Investors & media

Doto Medical LLC is a European medtech company aiming to improve the quality of life and survival of patients with chronic kidney disease. We engage the aptamer-driven therapeutic engine to bring hemodialysis to the next stage and develop it into a targeted therapy.
Key info
Product & value
Doto Medical LLC is a European medtech company aiming to improve the quality of life and survival of patients with chronic kidney disease. We engage the aptamer-driven therapeutic engine to bring hemodialysis to the next stage and develop it into a targeted therapy.
The aim of the PB103 program of Doto Medical is to develop an Adjunctive Blood Detoxification device (ABD), used as an add-on to standard hemodialysis therapy (see Technology). A device is to be connected to the heamodialysis device to work in-line during the hemodialysis procedure for a CKD patient, in a hospital/dialysis center setting. Thanks to the use of the patented PureApta™ technology and the highly specific aptamers obtained with its help, it will be possible to remove toxins that are not effectively removed during conventional hemodialysis, and which play a key role in the pathogenesis of CKD, progressive degradation of kidney function, development of cardiovascular complications or excessive activation of the immune system (see Portfolio). The use of a highly selective method will avoid the removal of molecules beneficial to the patient’s body, which is a significant advantage over similar products currently available on the market.
The ABD technology that selectively and efficiently removes unwanted and toxic molecules from the blood of CKD patients, which cannot be removed by standard hemodialysis, will allow doctors to:
The PB103 program is comprised of 3 sub-projects, each of which will develop a distinct adsorber aimed at achieving a different therapeutic effect by capturing a distinct set of molecular targets (see Portfolio). The work will therefore result in three marketable products, differing in the aptamers and the toxins captured, used by the ABD device, and targeting distinct clinical outcomes for specific clinical presentations of CKD. The first of the products, aimed at maintaining residual kidney activity, will be ready to start patient trials in 2026, and the other two products will follow within a year.
Competitive edge
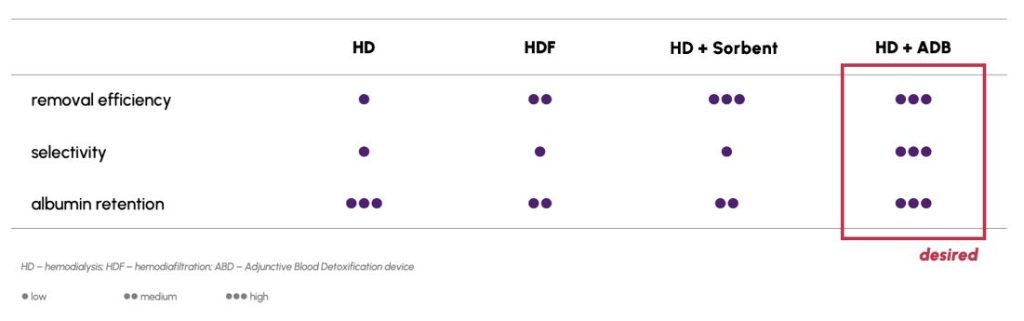
With our products, we offer a clear competitive edge: a technology that selectively and efficiently removes unwanted molecules from the blood of dialysis patients. A feature of the adsorbers provided in the ABD devices that distinguishes them from the competitive solutions is their high selectivity. The products available on the market not only remove harmful substances, but also other – often very important and necessary – plasma components, including e.g. immunoglobulins or albumin. In addition, adsorbers present on the market are characterized by limited efficiency of removing toxins. This means that the pool of toxins removed during the procedure is insufficient to prevent progressive tissue and organ damage in patients.
Our solution will allow the removal of selected toxins that play a key role in progressive CKD. It will be possible to lower their concentrations to physiological concentrations while maintaining homeostasis of the organism.
Market perspective
The size of the global hemodialysis market was valued at 78.30 billion USD in 2019 and is expected to reach 116.66 billion USD by 2030, with a CAGR of 3.60% in the years 2020-2030. In 2020, North America had the largest share of the global hemodialysis market, followed by Europe and the Asia-Pacific region.
The factors driving the growth of the market are the growing number of patients with end-stage renal disease, the lack of availability of kidneys for transplantation, as well as the increase in the incidence of diabetes and hypertension, which are key risk factors for the development of kidney diseases. According to a 2020 report by the International Society of Nephrology (ISN), the global burden of chronic kidney disease continues to grow and is projected to become the fifth leading cause of death worldwide by 2040. Moreover, the outbreak of the COVID-19 pandemic has increased the need for dialysis around the world. This has brought regulatory support from various governmental bodies to the market and increased financial incentives. These factors are expected to contribute to the growth of the market in the coming years.
Several key players are introducing technologically advanced products to achieve a competitive advantage in the industry. It is expected that such activities will also accelerate market growth during the forecast period.
Growth potential
Our current portfolio aims at the development of an ABD device as an add-on to the hemodialysis procedure in patients with chronic kidney disease.
Another area where the proposed solution could be applied is continuous renal replacement therapy. It is carried out in intensive care units in patients with acute renal failure and other organ failure, which is most often a consequence of severe sepsis and septic shock, but also large blood loss after traffic accidents or surgical procedures.
Moreover, thanks to the developed aptamer selection technology and optimization of the adsorber production process, it will be possible to further develop extracorporeal therapies to remove of other substances from the patient’s body. The use of aptamers offers a wide range of possibilities in terms of specific capture of harmful particles produced by the body or of external origin.